Musculoskeletal disorders such as joint pain, arthritis, and spinal deformities including scoliosis represent a significant global health burden, particularly with advancing age. These conditions are closely associated with chronic low-grade inflammation of muscles, joints, connective tissue, and the skeletal system. Aging induces molecular, cellular, biomechanical, and metabolic changes that predispose individuals to progressive musculoskeletal degeneration. This article explores the scientific basis of age-related inflammation and its role in the development and progression of orthopedic disorders, arthritis, joint pain, and scoliosis, with a focus on inflammatory mechanisms, muscle-joint interactions, and spinal biomechanics.
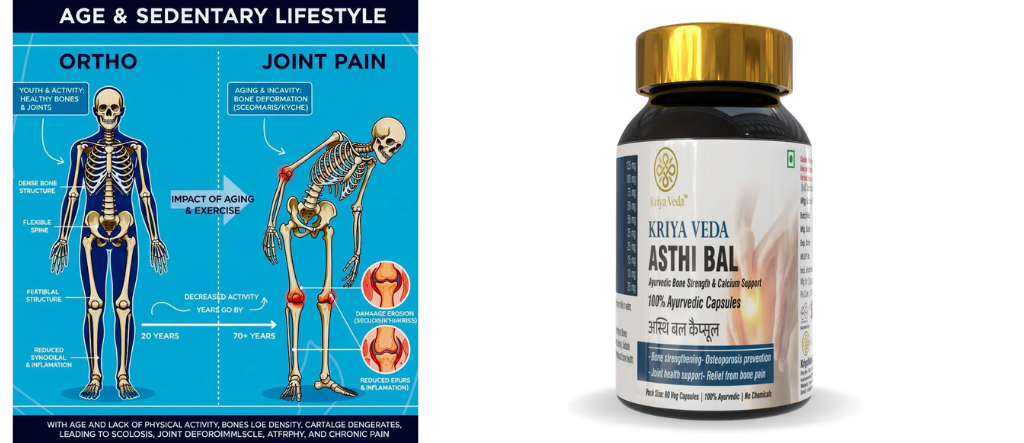
The musculoskeletal system undergoes continuous remodeling throughout life. With aging, this balance between tissue regeneration and degeneration becomes impaired, leading to increased susceptibility to orthopedic disorders. Joint pain, osteoarthritis, inflammatory arthritis, and spinal abnormalities such as scoliosis often emerge or worsen with age due to cumulative mechanical stress, cellular senescence, hormonal decline, immune dysregulation, and chronic inflammation.
Age-related inflammation, often termed “inflammaging,” plays a central role in musculoskeletal degeneration. It contributes to muscle weakness, cartilage breakdown, joint stiffness, altered posture, and spinal deformities.
2. Aging and Musculoskeletal Inflammation (Inflammaging)
2.1 Concept of Inflammaging
Inflammaging refers to a state of persistent, low-grade systemic inflammation that increases with age. It is characterized by elevated circulating levels of pro-inflammatory cytokines such as:
• Tumor necrosis factor-α (TNF-α)
•Interleukin-6 (IL-6)
•Interleukin-1β (IL-1β)
•C-reactive protein (CRP)
These mediators negatively affect muscles, joints, cartilage, tendons, and bones, accelerating tissue degeneration and pain.
2.2 Cellular and Molecular Mechanisms
Key age-related changes include:
•Accumulation of senescent cells producing inflammatory mediators
•Reduced mitochondrial function leading to oxidative stress
•Impaired autophagy and tissue repair mechanisms
•Dysregulated immune responses
Together, these changes create a chronic inflammatory microenvironment within musculoskeletal tissues.
3. Muscle Inflammation and Its Role in Joint and Spinal Disorders
3.1 Sarcopenia and Muscle Inflammation
Aging is associated with sarcopenia, the progressive loss of muscle mass and strength. Chronic inflammation contributes to:
•Muscle fiber atrophy
•Reduced satellite cell activity
•Increased muscle stiffness and fibrosis
Inflamed and weakened muscles fail to provide adequate joint stabilization, leading to abnormal load distribution across joints and the spine.
3.2 Muscle-Joint Interaction
Muscles act as dynamic shock absorbers and stabilizers. When inflamed or weakened:
•Joint compression increases
•Cartilage wear accelerates
•Pain perception intensifies due to sensitized nociceptors
This muscle-joint dysfunction is a critical contributor to age-related joint pain and arthritis.
4. Joint Pain and Age-Related Degeneration
4.1 Pathophysiology of Joint Pain
Joint pain in aging individuals arises from:
•Synovial inflammation
•Cartilage degradation
•Subchondral bone remodeling
•Ligament and tendon degeneration
Inflammatory mediators stimulate pain receptors and degrade extracellular matrix components, leading to stiffness, swelling, and reduced mobility.
4.2 Role of Mechanical Stress
Cumulative mechanical stress from daily activities, poor posture, obesity, and reduced muscle support amplifies inflammatory responses within joints, particularly in weight-bearing joints such as the knees, hips, and spine.
5. Arthritis and Age-Related Inflammation
5.1 Osteoarthritis
Osteoarthritis (OA) is the most prevalent age-related joint disorder. It is no longer considered purely a “wear-and-tear” disease but a chronic inflammatory condition involving:
•Cartilage breakdown due to matrix metalloproteinases
•Synovial inflammation
•Low-grade immune activation
Inflammation disrupts cartilage homeostasis, impairing its ability to withstand mechanical loads.
5.2 Inflammatory Arthritis
Conditions such as rheumatoid arthritis may also worsen with age due to immune dysregulation. Chronic inflammation leads to:
•Joint erosion
•Deformities
•Progressive disability
Aging immune systems exhibit reduced tolerance, increasing susceptibility to autoimmune joint damage.
6. Scoliosis and Age-Related Musculoskeletal Changes
6.1 Degenerative Scoliosis
Adult and age-related scoliosis, often termed degenerative scoliosis, develops due to:
•Asymmetric degeneration of intervertebral discs
•Facet joint arthritis
•Muscle imbalance and weakness
•Chronic inflammation of paraspinal muscles
These changes alter spinal alignment, leading to lateral curvature and rotational deformity.
6.2 Role of Muscle Inflammation in Spinal Deformity
Inflammation and weakness in spinal stabilizing muscles (paraspinals, core muscles) reduce spinal support. Uneven muscle pull and joint degeneration contribute to progressive curvature, pain, and reduced spinal flexibility.
7. Systemic Factors Aggravating Age-Related Inflammation
Several systemic factors exacerbate musculoskeletal inflammation with age:
•Hormonal decline (estrogen, testosterone, growth hormone)
•Poor nutrition and micronutrient deficiencies
•Sedentary lifestyle
•Chronic metabolic conditions (diabetes, obesity)
•Gut microbiota imbalance influencing immune regulation
These factors collectively intensify inflammation and slow tissue repair.


